
It is mostly based on tables provided by NIST. The following list includes the metallic elements of the first six periods.
#POTASSIUM REACTIVITY SERIES#
The reactivity series is sometimes quoted in the strict reverse order of standard electrode potentials, when it is also known as the " electrochemical series". Copper and silver will react with nitric acid but because nitric acid is an oxidizing acid, the oxidizing agent is not the H + ion as in normal acids, but the NO 3 − ion.Ĭomparison with standard electrode potentials Magnesium, aluminium and zinc can react with water, but the reaction is usually very slow unless the metal samples are specially prepared to remove the surface layer of oxide which protects the rest of the metal. There is some ambiguity at the borderlines between the groups. Metals in the middle of the reactivity series, such as iron, will react with acids such as sulfuric acid (but not water at normal temperatures) to give hydrogen and a metal salt, such as iron(II) sulfate:įe (s) + H 2SO 4 (l) → FeSO 4 (aq) + H 2 (g) The most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide:Ģ Na (s) + 2 H 2O (l) →2 NaOH (aq) + H 2 (g) There is no unique and fully consistent way to define the reactivity series, but it is common to use the three types of reaction listed below, many of which can be performed in a high-school laboratory (at least as demonstrations).
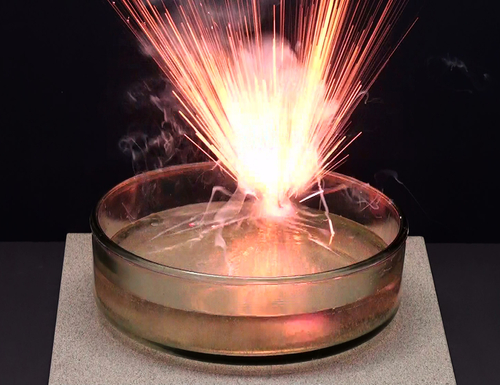
Or less commonly other alkali metals, hydrogen or calcium in the Kroll process Pyrometallurgical extraction using magnesium, In boiling water, and very vigorously with acids Reacts very slowly with cold water, but rapidly

It is used to summarize information about the reactions of metals with acids and water, single displacement reactions and the extraction of metals from their ores. In chemistry, a reactivity series (or activity series) is an empirical, calculated, and structurally analytical progression of a series of metals, arranged by their "reactivity" from highest to lowest. Not to be confused with Electrochemical series.


 0 kommentar(er)
0 kommentar(er)
